Kyleena satisfaction study
KYSS is a prospective, multinational, single-arm, observational study.
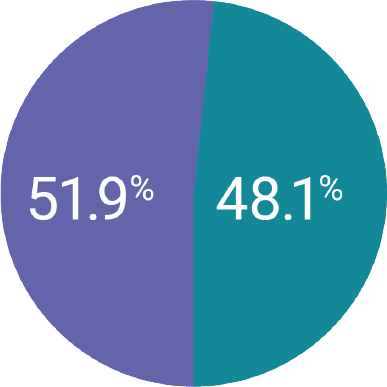
Kyleena® placement was attempted in 1,129 women and successful in 1,126 women1
Adapted from Stovall DW et al 2021
The majority of women were between 18 and 35 years of age (67.1%, n=756).
Participants were recruited from Belgium, Canada, Germany, Mexico, Norway, Sweden, Spain and the USA, from 2017 to 2018.1
- Either a COC or a POP.1 Return to content
- "Other" includes all methods with <10% of women using them: vaginal HC (3.1%), non-hormonal IUD (3.0%), natural planning (1.2%), implant (0.7%), Injectables, transdermal HC (0.2%) and backup contraception (0.09%).1 Return to content
COC – combined oral contraceptive; HC – hormonal contraception; IUD – intrauterine device; IUS – intrauterine system; KYSS – Kyleena Satisfaction Study;
POP – progestogen-only pill
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 Return to content
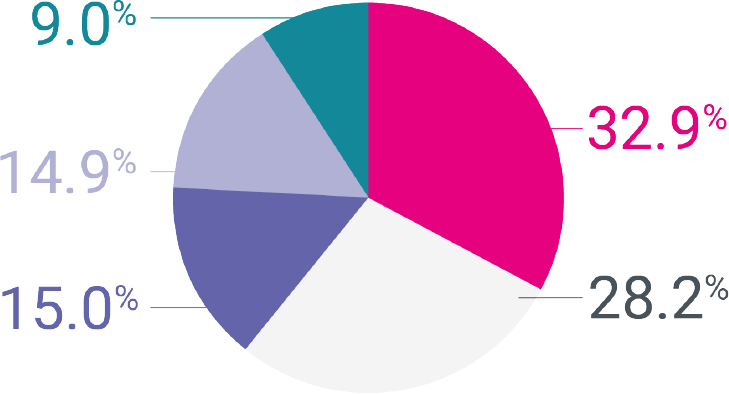
Women want more from their contraception than just clinical efficacy1

No contraceptive routine
Expectation of improved
bleeding pattern†
Low hormone dose
High contraceptive
reliability
No contraceptive routine
Expectation of improved
bleeding pattern†
Low hormone dose
High contraceptive
reliability
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 Return to content
Kyleena: placement rated as easy for most women regardless of parity2
87%
of 491 nulliparous women in KYSS were satisfied with Kyleena®
85.1%
of 477 parous women in KYSS were satisfied with Kyleena®
Satisfaction was similarly high for both parous and nulliparous women1
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 [DOI: 10.1080/13625187.2021.1975268. Online ahead of print]. Including supplementary appendix Return to content
- Beckert V et al. Eur J Contracept Reprod Health Care 2020;25:182–189. Return to content
- Black K et al. Eur J Contracept Reprod Health Care 2012;17(5):340–348. Return to content
- Daniele MAS et al. Reprod Health 2017;14:119. Return to content
- Akdemir Y and Karadeniz M. Eur J Contracept Reprod Health Care 2019;24(3):240–245 Return to content
Kyleena®: high satisfaction rates, irrespective of age1
Adapted from Stovall DW et al 2021
- Results shown represent women who reported being “very satisfied” or “Somewhat satisfied” with Kyleena® at the end of the final visit Return to content
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 Return to content
- Bayer PLC. Kyleena® Summary of Product Characteristics. Return to content
Most women were satisfied with Kyleena®,* no matter their…
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 Return to content
- Results shown represent women who reported being “very satisfied” or “somewhat satisfied” with Kyleena® at the end of the final visit.1 Return to content
- Results shown for each of the topics mentioned are representative of one of the subgroups only. Not all patients had the same previous contraception, motivation for choosing Kyleena®, or country of residency. Return to content
HC – hormonal contraception
Kyleena® demonstrated a high level of satisfaction with the bleeding profile in KYSS1
Bleeding profile satisfaction is important, as it can significantly impact women’s quality of life2-4
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 Return to content
- Schoep ME et al. BMJ Open 2019;9(6):026186. Return to content
- Schoep ME et al. AM J Obstet Gynecol 2019;220(6):569. Return to content
- Sveinsdóttir H. J Clin Nurs 2018;27(3–4):e503–e513 Return to content
- A total of 632/887 women in KYSS reported their satisfaction with their bleeding profile while on Kyleena®.1 Return to content
KYSS – Kyleena® Satisfaction Study
81.5% of patients in the patient safety analysis group experienced no adverse events with Kyleena®1
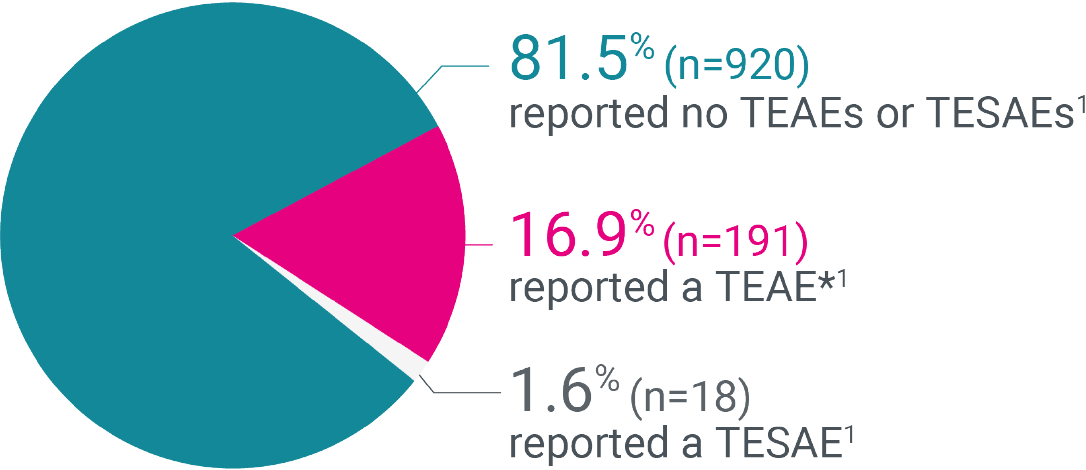
6.1%
(n=69)
and
0.4%
(n=4)
Discontinued Kyleena® due to TEAEs
and TESAEs, respectively1
- The most common TEAEs were gastrointestinal disorders 3.3%, reproductive system and breast disorders 9.5% and infections and infestations 2.3%1 Return to content
SAF – safety analysis set; TEAE – treatment-emergent adverse event; TESAE – treatment-emergent serious adverse event.
Please refer to the SmPC for the full summary of Kyleena's safety profile
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 Return to content
Few women discontinued Kyleena® in KYSS1
Discontinuation | Total (N=1,129) |
|---|---|
Kyleena® still in use at planned EoO | 919 (81.4) |
Kyleena® discontinued before planned EoO | 210 (18.6) |
Discontinuation due to TEAEs | 69 (6.1) |
Discontinuation due to TESAEs | 4 (0.4) |
Primary reason for discontinuation |
|
Unsuccessful Kyleena® placement attempt | 3 (0.3) |
Lost to follow-up | 105 (9.3) |
Expulsion of Kyleena® | 6 (0.5) |
Removal of Kyleena® | 94 (8.3) |
(Serious) adverse event | 62 (5.5) |
Pregnancy | 3 (0.3) |
Wish for pregnancy | 11 (1.0) |
Switch contraceptive methods | 6 (0.5) |
Dissatisfaction with Kyleena® | 9 (0.8) |
Investigator decision | 1 (0.09) |
Not specified | 0 (0.0) |
Adapted from Stovall DW et al 2021
EoO – end of observation period; KYSS – Kyleena® Satisfaction Study; SAF – safety analysis set;
TEAE – treatment-emergent adverse event; TESAE – treatment-emergent serious adverse event
KYSS: new perspectives on Kyleena® Summary
- Stovall DW et al. Eur J Contracept Reprod Health Care 2021 [DOI: 10.1080/13625187.2021.1975268. Return to content
- Beckert V et al. Eur J Contracept Reprod Health Care 2020;25:182–189. Return to content
Kyleena case studies
Reporting adverse events and quality complaints
If you want to report a side effect or quality complaint, please contact your health care professional (e.g. physician or pharmacist) or The Health Products Regulatory Authority, Reports can also be reported directly to Bayer through this link or by emailing directly on adr-reland@bayerhealthcare.com