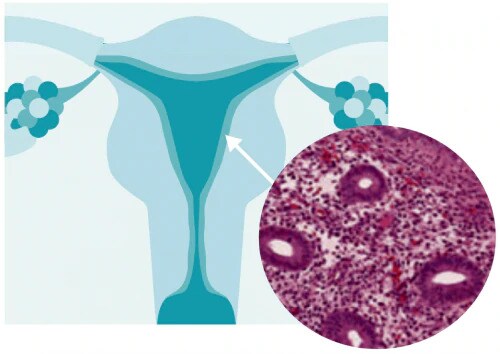
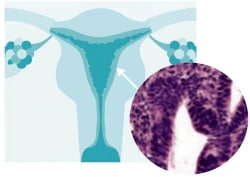
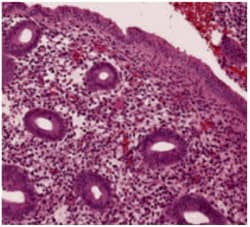
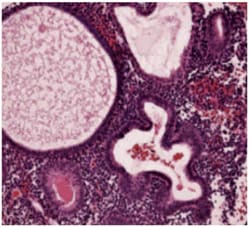
What is endometrial hyperplasia?
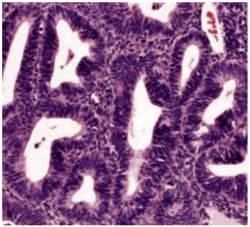
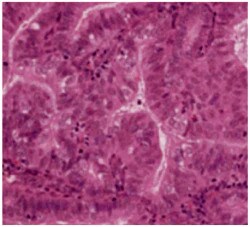
Endometrial hyperplasia is broadly defined as an excessive cellular proliferation leading to an increased volume of endometrial tissue. It is characterised by an increase in the endometrial gland-to-stroma ratio greater than 1:1.1
Endometrial hyperplasia is further classified as simple or complex, with or without atypia. This classification system is based on the complexity and crowding of the glandular architecture.1
The most common presenting symptom of endometrial hyperplasia is abnormal uterine bleeding, including:
However, endometrial hyperplasia can also be asymptomatic and can spontaneously regress without being detected.1
- Palmer JE, et al. Obstet Gynecol 2008;10211-216 Return to content
- RCOG/BSGE Management of Endometrial Hyperplasia [online] February 2016. Available from https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_67_endometrial_hyperplasia.pdf. Return to content
Histological classification of endometrial hyperplasia
Aetiology and risk factors
Oestrogen stimulates endometrial proliferation.1 A relative excess of oestrogen (exogenous or endogenous) compared with progesterone is considered to be one of the principle causes in endometrial hyperplasias.1
Key risk factors in post-menopausal women include:
Other risk factors include:
- Palmer JE, et al. Obstet Gynecol 2008;10211-216. Return to content
- Fu YS, et al. West J Med 1990;15350-61. Return to content
- Sanderson PA, et al. Hum Reprod Update 2017;23:232-254. Return to content
Incidence and diagnosis
- RCOG/BSGE Management of Endometrial Hyperplasie [online] February 2016. Available from https://www.rcog.org.uk/globalassets/documents/guidelines/green-top-guidelines/gtg_67_endometrial_hyperplasia.pdf. Return to content
- Chandra V, et al. J Gynecol Oncol 2016:27:eB. Return to content
The only levonorgestrel intrauterine system licensed for five years for endometrial protection during oestrogen replacement therapy (ERT)1

Efficacy and safety
The efficacy of Mirena® in preventing oestrogen-induced hyperplasia in peri-menopausal and postmenopausal women has been assessed in various studies.2,3

Mirena® significantly decreased menstrual bleeding compared with conventional oral hormone replacement therapy (P=0.001, N=200).2

Continuing Mirena® use during the transition from contraception to ERT has no adverse effects on the vaginal bleeding profile.5

The proportion of patients who had difficulties in coping with any items from the Women's Health Questionnaire decreased during both the contraception and ERT phases with Mirena®.5
- FSRH. Intrauterine Contraception [online] October 2015. Available from: https://www.fsrh.org/standards-and-guidance/documents/ceuguidanceintrauterinecontraception/. Return to content
- Boon J, et al. Maturitas 2003;46:69-77. Return to content
- Chandra V, et al. J Gynecol Oncol. 201627e8. Return to content
- Raudaskoski T, et al. BJOG 2002;109 136-144. Return to content
- Depypere H, et al. Eur J Obst Gyn Repr Biol 2010;153:176-18O. Return to content
Recommendations
Reporting adverse events and quality complaints
If you want to report a side effect or quality complaint, please contact your health care professional (e.g. physician or pharmacist) or The Health Products Regulatory Authority, Reports can also be reported directly to Bayer through this link or by emailing directly on adr-reland@bayerhealthcare.com